Astellas Pharma Inc. (TSE: 4503) (President and CEO: Naoki Okamura, "Astellas") and 4D Molecular Therapeutics, Inc. (NASDAQ: FDMT) (CEO: David Kirn, MD, "4DMT") today announced a license agreement under which Astellas gains rights to utilize the intravitreal retinotropic R100* vector invented by 4DMT for one genetic target implicated in rare monogenic ophthalmic disease(s), with options to add up to two additional targets implicated in rare monogenic ophthalmic diseases after paying additional option exercise fees.
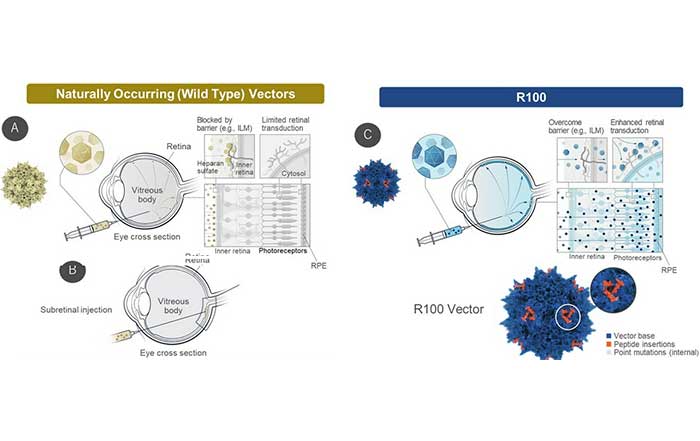
R100 is an adeno-associated virus (AAV) vector invented by 4DMT for intravitreal delivery. It has the ability to penetrate the internal limiting membrane barrier and to efficiently transduce the entire retina, resulting in robust transgene expression within retinal cells. All three 4DMT clinical-stage ophthalmic product candidates utilize the R100 vector, including 4D-150 for wet age-related macular degeneration and diabetic macular edema.
Under the terms of the agreement, 4DMT will provide its proprietary R100 vector technology to Astellas to deliver Astellas' unique genetic payloads for the treatment of rare monogenic diseases. Astellas will conduct all subsequent research, development, manufacturing, and commercialization activities. 4DMT will receive US$20 million upfront, and potential future option fees and milestones of up to US$942.5 million including potential near-term development milestones of US$15 million for the initial target. In addition, 4DMT is entitled to receive mid-single digit to double-digit, sub-teen royalties on net sales of all licensed products.
"This collaboration with Astellas, a leader in AAV gene therapy, continues to validate R100 for routine intravitreal low dose delivery of genetic payloads for the treatment of retinal diseases," said David Kirn, M.D., Co-Founder and Chief Executive Officer of 4DMT. "With over 70 patients dosed to-date with R100-based product candidates in wet age-related macular degeneration and rare ophthalmic diseases, this collaboration also demonstrates the modularity of the Therapeutic Vector Evolution platform resulting in efficient design and development of new intravitreal products. 4DMT retains rights to large market non-hereditary ophthalmic diseases."
Adam Pearson, Chief Strategy Officer (CStO) at Astellas said, "At Astellas, we have a strong commitment to developing novel treatments for ophthalmic diseases, and have positioned Blindness & Regeneration as one of the Primary Focuses of our R&D strategy. Staying at the forefront of gene therapy technology is a key part of our strategy. We believe that this collaboration will bring synergies between the two companies' cutting-edge research, and will ultimately lead to the development of new therapeutics for patients with ophthalmic diseases at high risk of blindness."
About Astellas
Astellas Pharma Inc. is a pharmaceutical company conducting business in more than 70 countries around the world. We are promoting the Focus Area Approach that is designed to identify opportunities for the continuous creation of new drugs to address diseases with high unmet medical needs by focusing on Biology and Modality. Furthermore, we are also looking beyond our foundational Rx focus to create Rx+® healthcare solutions that combine our expertise and knowledge with cutting-edge technology in different fields of external partners. Through these efforts, Astellas stands on the forefront of healthcare change to turn innovative science into VALUE for patients.